Abstract

The ongoing COVID-19 pandemic continues to raise challenges for safe administration of allogeneic hematopoietic stem cell transplantation (allo-HSCT). From March to August 2020, unrelated donor (URD) peripheral blood stem cell (PBSC) products were shipped to central national hubs and cryopreserved either at the site of collection or infusion, a shift from the traditional practice of infusing fresh stem cells. The National Marrow Donor Program (NMDP) instituted this mandate due to uncertainty around international transportation of stem cells as well as a fail-safe to prevent interruptions to a planned transplant if a donor contracted COVID-19 after the recipient started conditioning chemotherapy. We previously published our single institution experience demonstrating equivalent short-term clinical outcomes (overall survival [OS], progression free survival [PFS], relapse, and non-relapse mortality [NRM]) between adult recipients of fresh vs cryopreserved allogeneic stem cells (median follow up time 16 months [fresh] vs 8 months [cryopreserved]) (PMID: 34581754). We also demonstrated lower T-cell chimerism at day 30 and 100 after receipt of cryopreserved grafts. There is a paucity of data evaluating longer term outcomes of patients receiving cryopreserved stem cells compared to fresh.
Currently there is variability among donation and transplant centers around the practice of cryopreservation prior to allo-HSCT. With ongoing surges of COVID-19 and evolution of new variants, the risk of donors or recipients contracting COVID-19 leading to delays or disruptions in planned allo-HSCT remains high. In January 2022 with the rise of the Omicron variant of COVID-19, the NMDP reinstated the cryopreservation recommendation. Anecdotal reports indicate multiple recent instances of a planned URD testing positive for COVID-19 after conditioning chemotherapy had started but before stem cell harvest, raising the potential for severe deleterious outcomes in the intended transplant recipient.
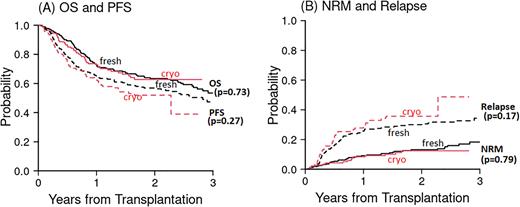
Given the unremitting COVID-19 surges three years into the pandemic and continued uncertainty around longer-term survival and relapse rates of allo-HSCT using cryopreserved stem cells, we reanalyzed our prior cohort and added 116 additional patients (40 cryopreserved, 76 fresh) with at least 1-year follow up for a total of 141 and 279 patients receiving cryopreserved versus fresh (respectively) URD PBSCs between January 1, 2019, and July 31, 2021. The cohorts had similar baseline characteristics including donor/recipient age/sex, disease, and conditioning regimen/intensity. Patients receiving cryopreserved cells were more likely to receive post-transplant cyclophosphamide as part of the graft versus host disease (GVHD) prophylaxis regimen (24% vs 15%, p=0.015). Median follow-up time among survivors in this cohort was 22 months (range, 4-41 months). Two-year OS was not different in patients receiving fresh versus cryopreserved stem cells (63% vs 63%, p=0.73) (Figure 1A). Two-year PFS was also similar (57% for fresh, 52% for cryopreserved, p=0.27) (Figure 1A). Relapse (36% vs 30%, p=0.17) and NRM (12% vs 13%, p=0.79) at two years were comparable between patients receiving fresh versus cryopreserved stem cells (Figure 1B). In total, 8 patients died of COVID-19 in this cohort (4 fresh, 3 cryopreserved). Cumulative incidence of moderate/severe chronic GVHD (cGVHD) was higher in patients receiving fresh stem cells versus cryopreserved (24% vs 9%, p=0.0003). Whereas cryopreservation was associated with lower T-cell chimerism early after transplant, by 1-year post-transplant, T-cell chimerism was not different (2% vs 5% of fresh vs cryopreserved patients with T-cell chimerism <75% at 1 year, p=0.14). However, Day 30 T-cell chimerism <75% was associated with a reduction in OS and PFS regardless of cryopreservation of stem cells (data not shown).
As the COVID-19 pandemic continues with frequent surges in case numbers and rapid viral evolution, the risk for interruptions in life-saving allo-HSCT therapies remains high. Our data show that allo-HSCT with cryopreserved URD PBSCs results in similar OS, PFS, relapse rates, and NRM at 2 years post-transplantation. We found that cryopreservation is associated with lower 1-year cumulative incidence of moderate/severe cGVHD without compromise in OS. While this is an intriguing finding, larger multicenter analyses are needed to confirm these observations.
Disclosures
Cutler:Janssen: Consultancy; Incyte: Consultancy; Sanofi: Consultancy; Jazz: Consultancy; Deciphera: Consultancy; Editas: Consultancy; Pfizer: Consultancy; CareDx: Consultancy; Mallinckrodt: Consultancy; BMS: Consultancy; CTI BioPharma: Consultancy; Equilium: Consultancy; Cimeio: Current equity holder in private company; Omeros: Consultancy. Ritz:Equillium: Research Funding; Gadeta: Research Funding; Kite/Gilead: Research Funding; Oncternal: Research Funding; Novartis: Research Funding; Akron Biotech: Consultancy, Membership on an entity's Board of Directors or advisory committees; AvroBio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Clade Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Draper: Consultancy, Membership on an entity's Board of Directors or advisory committees; Garuda Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; LifeVault Bio: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Smart Immune: Consultancy, Membership on an entity's Board of Directors or advisory committees; Talaris Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; TScan Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees. Shapiro:Miltenyi: Honoraria. Romee:Glycostem Therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Scientific Advisory Board; Skyline Therapeutics: Research Funding; Crispr Therapeutics: Research Funding; xNK therapeutics: Membership on an entity's Board of Directors or advisory committees, Other: Scientific Advisory Board; InnDura Therapeutics: Current equity holder in publicly-traded company. Wu:BioNTech: Current equity holder in publicly-traded company; Pharmacyclics: Research Funding. Nikiforow:Kite, a Gilead Compnay: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Soiffer:Be The Match/National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Juno: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy; VOR Biopharma: Consultancy; Jazz: Consultancy; Rheos Therapeutics: Consultancy; Alexion: Consultancy; Kiadis: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal